Guidelines for Cleaning and Preparing External- and Internal-Use Ultrasound Transducers and Equipment Between Patients as Well as Safe Handling and Use of Ultrasound Coupling Gel, 2025 Revision
Jul 2, 2025
SUMMARY
Adequate transducer preparation is mandatory to protect patients from potential infection. The level of preparation depends on the type of examination performed. Preparation of external transducers between patients requires a low-level disinfection (LLD) process. Preparation of internal transducers between patients requires routine mandatory high-level disinfection (HLD) and the use of a high-quality single-use transducer cover during each examination. Users should consult a transducer’s manufacturer instructions for disinfecting devices. Barriers used for internal and interventional percutaneous procedures must be single-use transducer covers that meet the sterility requirements of the procedure. For all chemical disinfectants, precautions must be taken to protect workers and patients from the toxicity of the disinfectant. Sterile single-use gel packets should be used when infection is a concern. If cross-contamination is a concern, non-sterile single-use gel packets should be used. For all other situations, multidose containers may be used. Dry heat should be used to heat gel where medically needed. LLD cleaning of equipment is a significant part of interrupting the cross-contamination chain. The cleaning frequency is dictated by operator and patient exposure to microbial activity.
Following guidelines other than Ultrasound Original Equipment Manufacturers’ (OEM) instructions for use (IFU) may lead to transducer damage1 and may negatively affect diagnostic results.2,3 Regular interval inspection of ultrasound scanners and transducers (connector, cable, housing, acoustic lens) is recommended to ensure performance. Imaging with a tissue-mimicking phantom may help reveal imaging degradation.
The AIUM does not endorse or promote any specific commercial products. It is the responsibility of each entity to follow transducer manufacturer guidelines (for device-agent compatibility) and applicable infection control recommendations (such as these guidelines).
SECTIONS
1. Procedural guidelines for transducer cleaning and preparation
2. New literature and other relevant guidelines
3. Safe handling and use of ultrasound coupling gel
4. Safe handling of ultrasound scanners and other equipment
5. Prevent misdiagnosis due to the use of damaged ultrasound transducers
SECTION I: PROCEDURAL GUIDELINES FOR TRANSDUCER CLEANING AND PREPARATION
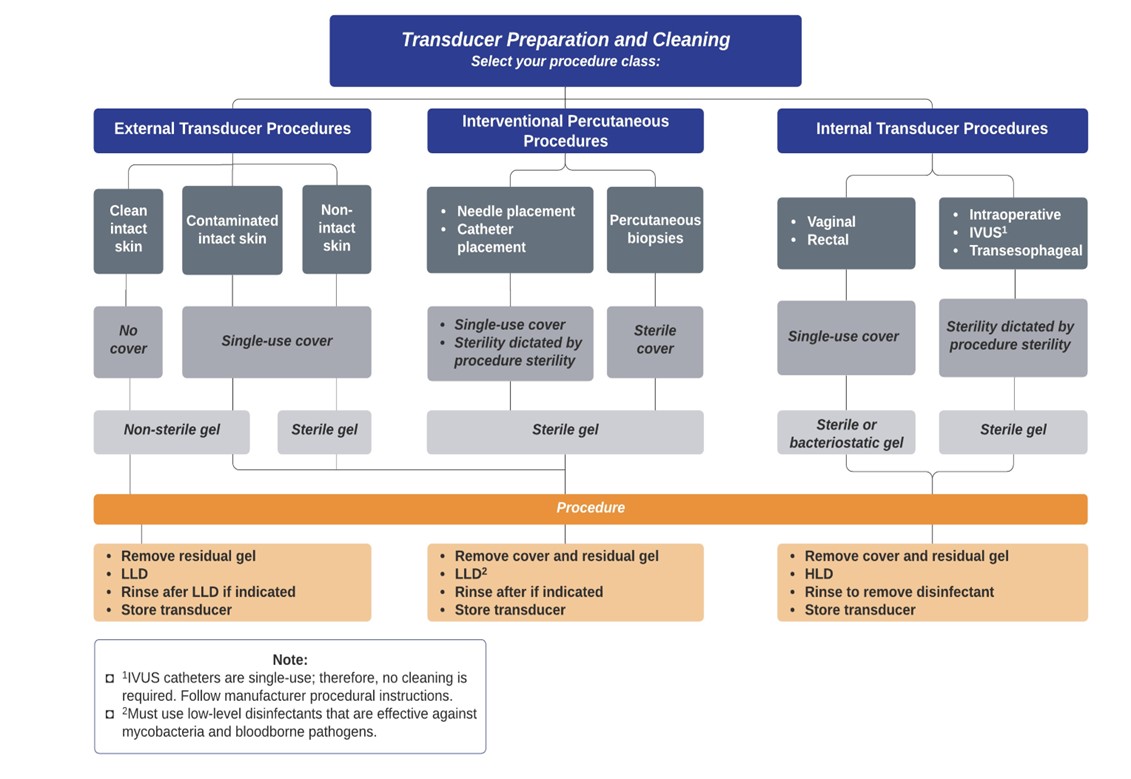
Procedural Graphics (click image to enlarge)
Introduction
The purpose of the first section of this document is to provide guidance regarding the cleaning and preparation of ultrasound transducers. Some manufacturers use the term “probes” or “imaging arrays”.
Medical instruments fall into different categories with respect to their potential for pathogen transmission. Therefore, different types of medical instruments require different cleaning methods. The most critical instruments are those that are intended to penetrate skin or mucous membranes, such as scissors, forceps, tweezers, or hemostats. These require sterilization. Less critical instruments (often called “semicritical” instruments) that simply come into contact with mucous membranes, such as endocavitary transducers, require HLD rather than sterilization. “Noncritical” devices that come into contact with intact skin but not mucous membranes, such as blood pressure cuffs, a stethoscope, or external transducers, require LLD.
- External transducers that only come into contact with clean, intact skin are considered noncritical devices and should be cleaned after every use, as described below in number 1.
- External transducers that come into contact with contaminated skin (such as skin infections) should be covered with a single-use transducer cover.
- Transducers for interventional percutaneous procedures that are used for percutaneous needle or catheter placement, such as vascular access, thoracentesis, paracentesis, arthrocentesis, pericardiocentesis, lumbar puncture, or ultrasound-guided regional/local anesthesia, should be cleaned using LLD and be used in conjunction with a single-use transducer cover. Such a cover must warrant protection against human viruses, including human immunodeficiency virus, human papillomavirus (HPV), hepatitis B, and others of clinical significance. The level of transducer cover sterility is dictated by the level of procedure sterility. As examples, clean procedures requiring nonsterile transducer covers include peripheral vascular intravenous line placement, whereas full sterility procedures requiring full sterile transducer covers include percutaneous biopsies. Transducer covers can be commercial transducer sheaths or condoms as long as they fulfill institutionally defined infection control guidelines and procedure sterility requirements. If contamination of covered transcutaneous transducers with blood or other bodily fluids occurs, see steps in paragraph 2a of the next section.
- Internal transducers should be covered with a single-use transducer cover, as described below, when feasible. The level of transducer cover sterility is dictated by the level of procedure sterility. These transducers are therefore classified as semicritical devices.
Ultrasound Transducer Cleaning and Preparation
The following specific recommendations are made for the cleaning and preparation of all ultrasound transducers. For the protection of the patient and the health care worker, all internal examinations should be performed with the operator properly gloved throughout the procedure. Transducers need to be cleaned after each exam by using all of the following steps proposed by Abramowicz et al4:
1. Cleaning of all transducers—Disconnect the transducer from the ultrasound scanner as appropriate. After removal of the transducer cover (when applicable), remove bulk gel or debris from the transducer. Consider the use of a small brush, especially for crevices and areas of angulation, depending on the design of the particular transducer. Use a damp gauze pad or other soft cloth and a small amount of mild nonabrasive liquid soap, e.g., household dishwashing liquid or use a wipe to remove any remaining gel (film).
2. Disinfection can be (a) low-level or (b) high-level:
a. Disinfection of all transducers in external procedures, as well as interventional percutaneous procedures, should undergo LLD. If contamination of covered transcutaneous transducers with blood or other bodily fluids occurs, it can be eliminated with low-level disinfectants that are effective against mycobacteria and bloodborne pathogens (including hepatitis B virus, hepatitis C virus, and HIV).5–9 Human hands are always cleaned with LLD and covered with gloves.10
b. Disinfection of all internal transducers (e.g., transvaginal, transrectal, and transesophageal transducers), as well as intraoperative transducers, require HLD before they can be used on another patient. High-level chemical disinfectants rely on clean and dry surfaces as wet surfaces dilute the disinfectant; therefore, always dry the transducer before performing HLD. Chemical sterilants used for shorter exposure periods for internal transducer disinfection can also be part of the HLD process per CDC guidelines.5
Note: An obvious disruption in transducer cover integrity does not require modification of this protocol. Because of the potential disruption of the barrier sheath, HLD with chemical agents is necessary. The guidelines herein take into account possible transducer contamination due to a disruption in the barrier sheath.
3. Rinsing—Depending on the employed disinfection agent, the transducer should be thoroughly rinsed and dried after disinfection, following manufacturer guidelines. Potable water should be used for rinsing.
4. Storing—Transducers need to be stored in accordance with their disinfection level.
5. Remove gloves, dispose, and wash hands.
High-level liquid disinfection is required to ensure further statistical reduction in the microbial load. Examples of such high-level disinfectants are listed in Table 1. A complete list of Food and Drug Administration (FDA)-cleared liquid sterilants and high-level disinfectants is available online,2 and other agents are under investigation. To achieve HLD, the practice must meet or exceed the listed “High-Level Disinfectant Contact Conditions” specified for each product. Users should be aware that not all approved disinfectants on this list are safe for all ultrasound transducers.
Immersion of transducers in fluids requires attention to the individual device’s ability to be submerged. Although some transducers as well as large portions of the cable may safely be immersed up to the transducer’s connector, only the transducers of others may be submerged. Some manufacturers also note that the crystals of the array may be damaged if the transducer rests or impacts the bottom of the container, instead of being suspended in the disinfectant. Before selecting a method of disinfection, consult the manufacturer’s guidelines regarding the compatibility of potential disinfecting agents with a specific transducer. Relevant information is available online and in device manuals. Additionally, not all transducers can be cleaned with the same cleaning agents. Although some agents are compatible with all transducers of a given manufacturer, others must be limited to a subset of transducers.
The CDC recommends environmental infection control in the case of Clostridium difficile, consisting of “meticulous cleaning followed by disinfection using hypochlorite-based germicides as appropriate.”11 A hydrogen peroxide nanodroplet emulsion might provide an effective high-level disinfectant without toxicity.
The Occupational Safety and Health Administration as well as the Joint Commission (Environment of Care Standard IC 02.02.01 EP 9) have issued guidelines for exposure to chemical agents, which might be used for ultrasound transducer cleaning. Before selecting a high-level disinfectant, users should request the Material Safety Data Sheet for the product and make sure that their facility is able to meet the necessary conditions to minimize exposure (via inhalation, ingestion, or contact through skin/eyes) to potentially dangerous substances. Proper ventilation, a positive-pressure local environment, and the use of personal protective devices (e.g., gloves and face/eye protection) may be required.
Low Resource Settings
Users may be in settings, where resources are limited, and thus, cleaning and disinfection cannot be executed as laid out herein. These settings may include pandemics, supply chain disruptions and resource-disadvantaged regions. Providers are encouraged to execute guidelines to the extent possible given their setting. Dissemination of cleaning guidelines is essential and so is their proper execution.11 Providers should weigh the benefit of use of ultrasound for imaging or guidance, and the eventual risk that is introduced due to cleaning and disinfection at a lower level than recommended. If LLD agents are depleted, additional cleaning should use soap and (potable) water.12 However, this does not complete the disinfection. If transducer covers are indicated but are not available, medical gloves or other physical barriers (e.g., compatible medical dressings) should be used.
Table 1. Sterilants and High-Level Disinfectants Listed by the FDA
| Name | Composition/Action |
|---|---|
| Glutaraldehyde | Organic compound (CH2(CH2CHO) 2) Induces cell death by cross-linking cellular proteins; usually used alone or mixed with formaldehyde |
| Hydrogen peroxide | Inorganic compound (H2O2) Antiseptic and antibacterial; a very strong oxidizer with oxidation potential of 1.8 V |
| Peracetic acid | Organic compound (CH3CO3H) Antimicrobial agent (high oxidation potential) |
| Ortho-phthalaldehyde | Organic compound (C6H4(CHO)2) Strong binding to outer cell wall of contaminant organisms |
| Hypochlorite/hypochlorous acid | Inorganic compound (HClO) Myeloperoxidase-mediated peroxidation of chloride ions |
| Phenol/phenolate | Organic compound (C6H5OH) Antiseptic |
| Hibidil | Chlorhexidine gluconate (C22H30Cl2N10) Chemical antiseptic |
| Chlorine dioxide | Chlorine(IV) oxide (ClO2) |
| UV-C | Ultraviolet light as germicidal agent (wavelength 100 to 280 nm) |
Conclusions
The current literature points to the need for education on the proper use of transducer cleaning agents and procedures. It also points to the need of HLD for internal transducers (endocavitary) due to the risk of infection with HPV, for example. Contrary, use of external transducers on intact skin does not show an increased infection risk in conjunction with LLD. Prudent use of ultrasound includes guidance for interventional percutaneous procedures. In this case, the use of sterile gel and single-use protective covers (level of sterility dictated by the procedure sterility classification) justifies subsequent LLD, analogous to institutional health care guidelines for the use of gloves and LLD hand disinfection for medical personnel.
SECTION II: NEW LITERATURE AND OTHER RELEVANT GUIDELINES
Background
Kac et al13 examined endocavitary transducers and found them persistently contaminated despite the use of transducer covers. They concluded that transducers may carry pathogens, including HPV, unless properly disinfected between examination sessions. For disinfection, they recommend an antiseptic-impregnated towel as well as a type C ultraviolet light.
Adhikari et al14 compared infection rates between ultrasound-guided and traditionally placed peripheral intravenous lines. A nonsterile glove was used as a barrier between the ultrasound transducer and the patient, and coupling was achieved using a bacteriostatic lubricant. Transducers were cleaned between patients using LLD. They concluded that both showed low infection rates (0.52% ultrasound and 0.78% traditional; n = 402 each; P = .68) and that there was no increased infection rate with ultrasound guidance. The Spaulding classification15 is inconsistent with the results of this study.
Casalegno et al16 stated that a considerable number of endocavitary transducers are infected with high-risk HPV despite LLD and recommend that endocavitary transducers should be high-level disinfected (2.5% of transducers showed high-risk HPV after use and 1.8% before use; n = 198).
Westerway et al17 examined 171 swabs of transabdominal and transvaginal transducers, of which 60% and 14% were found to show evidence of bacterial contamination, respectively. After LLD, both showed approximately a 4% likelihood of contamination.
Westerway and Basseal11 also investigated if appropriate training was received and if cleaning procedures were followed. They found that 60% of respondents (n = 188) failed to receive adequate training before using any cleaning product. In addition, 33% had no access to written infection control policies for either transducers or ultrasound scanners (keyboard, connectors, cables, etc). Westerway and Basseal18 also investigated specifically the Australasian medical ultrasound practice. They found that only 10% of 392 users cleaned their keyboards after each patient. Fifty-six percent cleaned it once a day and 21% once per week.18 The machine cord was cleaned after each patient by 35% of all users (n = 393) and once a day by 32%. Fifteen percent cleaned it once a week. They concluded that education and updated guidelines may remedy this situation.
In 2013, Seki et al19 traced an institutional nosocomial outbreak of multidrug-resistant Pseudomonas aeruginosa to a transesophageal echocardiogram transducer, specifically a 5-mm defect on the insertion tube, 15 cm from the end of the device. While the transducer was cleaned by way of centralized sterilization, the mechanical defect retained bacterial contamination. No transducer sheath was used.
In the same year, Sartoretti et al20 compared the bacterial load on ultrasound transducers (n = 36), bus poles (n = 11), and toilets (n = 10). Before training, 53 colony-forming units (CFU) were found in cultures from transducers, while 0 CFU were found after training. Bus poles and toilets showed 28 CFU (P = .772) and 4 CFU (P = .055), respectively. Thus, training was key in improving healthcare-associated infections.
Gottlieb et al21 acknowledged that the use of ultrasound for intravenous line placement adds a potential source for infection. However, they concurrently highlighted studies concluding that ultrasound-guided peripheral intravenous line placement reduces the need for central venous catheter placement in up to 80% of patients.22–25
Abramowicz et al4 provided cleaning guidelines for transvaginal transducers on behalf of the World Federation for Ultrasound in Medicine and Biology Safety Committee. They listed several HLD methods. Of these methods, only chlorine dioxide and a vaporized hydrogen peroxide system have been reported to effectively remove HPV. However, the supporting evidence for the latter method was based on several studies paid for by grants from its manufacturer.
The Ultrasound Working Group of the European Society of Radiology released best practice recommendations for infection prevention and control in ultrasound.26 They stated that for ultrasound transducers with protective covers that are in contact with mucous membranes or any body fluids (including interventional procedures, injections, tissue sampling, use in the theater, etc.) require HLD.
Protective barriers, such as medical gloves, are regulated by an acceptable quality level (AQL). The AQL is the maximum percentage of defective items permitted in a regulated product. Hence, the AQL may also mean the acceptable quality limit. The Center for Devices and Radiological Health in the Office of Device Evaluation at the FDA uses the AQL to define the acceptance level for medical gloves (21CFR800.20).27 Similarly, condoms, which are also mechanical protective barriers, are regulated by the AQL, and the World Health Organization (WHO) provides an AQL to set limits for their quality.28 Practitioners should be aware that condoms have a 10-fold stricter AQL (0.25%, WHO) compared to standard examination gloves (2.5%, FDA). They even exceed the AQL of surgical gloves (1.5%, FDA). Users should be aware of latex sensitivity issues and have non–latex-containing barriers available. In addition, transducer covers with pore sizes of less than 30 nm are now available. They effectively block most viruses, including HPV. One should perform HLD of the internal transducer between each use and employ an adequate transducer cover as a protective barrier.
CDC Definitions
According to the CDC Guideline for Disinfection and Sterilization in Healthcare Facilities11:
“Cleaning is the removal of visible soil (e.g., organic and inorganic material) from objects and surfaces and normally is accomplished manually or mechanically using (potable) water with soap or enzymatic products. Thorough cleaning is essential before HLD and sterilization because inorganic and organic material that remains on the surfaces of instruments interfere with the effectiveness of these processes.”
“Disinfection describes a process that eliminates many or all pathogenic microorganisms, except bacterial spores.”
Low-Level Disinfection (LLD)—Destruction of most bacteria, some viruses, and some fungi. Low-level disinfection will not necessarily inactivate Mycobacterium tuberculosis or bacterial spores.
Intermediate Level Disinfection (ILD)—Inactivation of M Tuberculosis, bacteria, most viruses, most fungi, and some bacterial spores.
High-Level Disinfection (HLD)—Destruction/removal of all microorganisms, except bacterial spores.
“Sterilization describes a process that destroys or eliminates all forms of microbial life and is carried out in healthcare facilities by physical or chemical methods. Steam under pressure, dry heat, ethylene oxide (EtO) gas, hydrogen peroxide gas plasma, and liquid chemicals are the principal sterilizing agents used in health care facilities. When chemicals are used to destroy all forms of microbiologic life, they can be called chemical sterilants. These same germicides used for shorter exposure periods also can be part of the disinfection process (i.e., HLD).”11
Intersocietal Position Statement
Several organizations addressed the issue of disinfection of transcutaneous ultrasound transducers used for percutaneous procedures or for the purpose of monitoring other invasive procedures. They recommend cleaning and LLD (including disinfectants that are effective against mycobacteria and bloodborne pathogens) for the reprocessing of transducers used for the above purposes.29
SECTION III: SAFE HANDLING AND USE OF ULTRASOUND COUPLING GEL
Background
Infection control is an integral part of the safe and effective use of ultrasound in medicine. For example, guidelines are in place for transducer disinfection to reduce the risk of iatrogenic and nosocomial infections. Although aspects of ultrasound coupling gel management and administration have been implicated in outbreaks of nosocomial infections with a variety of pathogenic organisms, recommendations for reducing gel-related infections vary (see Clinician Outreach and Communication Activity safety communication and Health Canada alert in the “Related Websites” section).30
The purpose of this document is to provide guidance regarding ultrasound coupling gel use to minimize risks of iatrogenic or nosocomial infections.31
Recommendations
Practices and institutions should adopt an infection control policy regarding the use of ultrasound coupling gel and ensure that appropriate staff are educated regarding this policy.
Sterile Gel
Sterile single-use gel packets are preferable to nonsterile gel when infection is a concern. Such situations include but are not limited to:
- All invasive procedures that pass a device through a tissue (e.g., needle aspiration, needle localization, and tissue biopsy);
- All ultrasound examinations performed on neonates; and
- All ultrasound examinations or procedures performed on nonintact skin or near fresh surgical sites.
Sterile or bacteriostatic gel should be considered for endocavitary examinations performed on intact mucous membranes (e.g., esophageal, gastric, rectal, and vaginal).
Nonsterile Gel
Single-use gel packets or multidose containers may be used.
If multidose containers are used, care should be taken to:
- Discard and replace multidose containers when empty; these should not be refilled;
- Appropriately seal the container when not in use; and
- Place gel bottle upright in bottle holder to avoid contact between the tip and other surfaces. Avoid direct contact between the gel container dispensing tip and any persons or instrumentation, including the ultrasound transducer.
If gel is to be used on a patient who is under droplet or contact precautions (such as COVID-19), discard the multidose container after use, or use a single-use gel packet.
Gel Warming
Dry heat should be the only method used to warm gel. Gel warmers should be cleaned and disinfected regularly according to the manufacturers’ and infection control policy’s requirements.
SECTION IV: SAFE HANDLING OF SCANNERS AND OTHER EQUIPMENT
Users should follow institutionally defined infection control guidelines for cleaning of ultrasound scanners, cables, connectors, and other equipment. The extent to which equipment needs to be cleaned is dictated by its exposure to the operator and the patient. Typically, this includes the ultrasound scanner console, its handles, the transducer cable and connector, gel containers, and the patient bed. Institutional infection control guidelines may also require a full scanner wipe down, e.g., for scanning in operating rooms. CDC guidelines for healthcare facilities (2017) address microbicidal activity of quaternary ammonium compounds (LLD) specifically for keyboards.32,33
Equally important to creating cleaning guidelines is their dissemination to and subsequent execution by the operators. Westerway and Basseal11 highlighted the lack of appropriate cleaning and disinfection training for a significant number of their survey responders. Approximately half of them did not undergo training for either purchased ultrasound equipment or cleaning products. Similar to this were the responses regarding actual equipment cleaning. Approximately 50% cleaned the transducer cord after each patient, but only 15% cleaned the keyboard. Most operators (57%) cleaned the keyboard daily, but 7.6% never did. Even in a non-critical setting, i.e., external transducers, not cleaning a keyboard is concerning. The cleaning frequency and level of sterility of ancillary equipment should follow institutional infection control guidelines for the procedure at hand and are dictated by operator and patient exposure to microbial activity. For example, for surgical procedures, equipment should be disinfected prior to the procedure (to prevent infection), whereas for COVID-19 patients, it should be disinfected after the procedure (to prevent cross-contamination).
SECTION V: PREVENTING MISDIAGNOSIS DUE TO THE USE OF DAMAGED ULTRASOUND TRANSDUCERS
Without proper care of ultrasound probes and equipment, ultrasound imaging performance can be reduced. One study found the performance of probes in routine clinical practice was compromised in some manner in 39.8% of the evaluated transducers.2 Another study, examining the failure rate of ultrasound systems in a hospital setting, showed that 12% of total failures were due to the ultrasound system and 88% were due to the transducer.1 It was also reported that lens delamination or some form of element damage in the transducer array caused transducer failures and compromised performance.2,3 Damaged transducers due to dead elements or elements with reduced sensitivity may reduce resolution and penetration, increase the noise floor, cause Doppler velocity errors, produce flow direction ambiguity, and increase spectral broadening.
One of the causes of damage associated with cleaning and disinfection in this study is the use of automated reprocessing devices that have not been validated by manufacturers. For this reason, researchers found that certain transducers, such as endocavitary transducers, had higher failure rates.
Using chemicals and reprocessing protocols or methods not validated by the responsible OEM may potentially damage a given transducer and present a patient safety issue or produce a non-diagnostic study. Even when following the cleaning, disinfection, and sterilization guidelines provided by various clinical societies, damage to ultrasound transducers may occur.1 It is essential, therefore, to both strictly follow the OEM transducer reprocessing guidance and inspect the transducer prior to clinical scanning for changes in visual appearance and function to ensure the transducer appears fully functional and clinically efficacious.
Reusing transducers that have been damaged during reprocessing will potentially negatively affect the acquired images. Echo contrast or flow velocity from these distorted images may provide inaccurate measurement results and misleading diagnosis.
References
- Hangiandreou NJ, Stekel SF, Tradup DJ, Gorny KR, King DM. Four-year experience with a clinical ultrasound quality control program. Ultrasound Med Biol 2011; 37:1350–1357.
- Vachutka J, Dolezal L, Kollmann C, Klein J. The effect of dead elements on the accuracy of Doppler ultrasound measurements. Ultrason Imaging 2014; 36(1):18–34.
- Weigang B, Moore GW, Gessert J, Phillips WH, Schafer M. The methods and effects of transducer degradation on image quality and the clinical efficacy of diagnostic sonography. J Diagnostic Med Sonography 2003; 19:3–13.
- Abramowicz JS, Evans DH, Fowlkes JB, et al. Guidelines for cleaning transvaginal ultrasound transducers between patients. Ultrasound Med Biol 2017; 43:1076–1079.
- Rutala W, Weber D. Guideline for Disinfection and Sterilization in Healthcare Facilities. Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/. Accessed March 23, 2020.
- Sehulster LM, et al. Guidelines for environmental infection control in health-care facilities. Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html. Accessed March 23, 2020.
- Morbidity and Mortality Weekly Report. 52(RR17); 62–64. Centers for Disease Control and Prevention website. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a2.htm. Accessed March 23, 2020.
- Occupational Safety and Health Standards. Occupational Safety & Health Administration website. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030. Accessed March 23, 2020.
- List B: EPA’s Registered Tuberculocide Products Effective Against Mycobacterium tuberculosis. United States Environmental Protection Agency website. https://www.epa.gov/sites/production/files/2020-09/documents/20200922listb.pdf. Accessed March 23, 2020.
- Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol 2002; 23(S12):S3–S40.
- Westerway SC, Basseal JM. The ultrasound unit and infection control - Are we on the right track? Ultrasound 2017; 25:53–57.
- Environmental Cleaning and Disinfection Recommendations. National Center for Immunization and Respiratory Diseases (NCIRD) DoVD website. https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/cleaning-disinfection.html. Accessed March 24, 2020.
- Kac G, Podglajen I, Si-Mohamed A, et al. Evaluation of ultraviolet C for disinfection of endocavitary ultrasound transducers persistently contaminated despite probe covers. Infect Control Hosp Epidemiol 2010; 31:165–170.
- Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med 2010; 29:741–747.
- Spaulding E. Chemical disinfection and antisepsis in the hospital. Hosp Res 1957; 9:5–31.
- Casalegno JS, Le Bail Carval K, Eibach D, et al. High risk HPV contamination of endocavity vaginal ultrasound probes: an underestimated route of nosocomial infection? PLoS One 2012; 7:e48137.
- Westerway SC, Basseal JM, Brockway A, Hyett JA, Carter DA. Potential infection control risks associated with ultrasound equipment - A bacterial perspective. Ultrasound Med Biol 2017; 43:421–426.
- Westerway SC, Basseal JM. Advancing infection control in Australasian medical ultrasound practice. Australas J Ultrasound Med 2017; 20:26–29.
- Seki M, Machida H, Yamagishi Y, Yoshida H, Tomono K. Nosocomial outbreak of multidrug-resistant Pseudomonas aeruginosa caused by damaged transesophageal echocardiogram probe used in cardiovascular surgical operations. J Infect Chemother 2013; 19:677–681.
- Sartoretti T, Sartoretti E, Bucher C, et al. Bacterial contamination of ultrasound probes in different radiological institutions before and after specific hygiene training: do we have a general hygienical problem? Eur Radiol 2017; 27:4181–4187.
- Gottlieb M, Sundaram T, Holladay D, Nakitende D. Ultrasound-guided peripheral intravenous line placement: a narrative review of evidence-based best practices. West J Emerg Med 2017; 18:1047–1054.
- Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med 2010; 28:1–7.
- Au AK, Rotte MJ, Grzybowski RJ, Ku BS, Fields JM. Decrease in central venous catheter placement due to use of ultrasound guidance for peripheral intravenous catheters. Am J Emerg Med 2012; 30:1950–1954.
- Shokoohi H, Boniface K, McCarthy M, et al. Ultrasound-guided peripheral intravenous access program is associated with a marked reduction in central venous catheter use in noncritically ill emergency department patients. Ann Emerg Med 2013; 61:198–203.
- Duran-Gehring P, Bryant L, Reynolds JA, et al. Ultrasound-guided peripheral intravenous catheter training results in physician-level success for emergency department technicians. J Ultrasound Med 2016; 35:2343–2352.
- Nyhsen CM, Humphreys H, Koerner RJ, et al. Infection prevention and control in ultrasound - best practice recommendations from the European Society of Radiology Ultrasound Working Group. Insights Imaging 2017; 8:523–535.
- Office USGP. 21 CFR 800.20 - Patient Examination Gloves and Surgeons’ Gloves; Sample Plans and Test Method for Leakage Defects; Adulteration. Government Printing Office website. https://www.govinfo.gov/app/details/CFR-2001-title21-vol8/CFR-2001-title21-vol8-sec800-20. Accessed March 24, 2020.
- Research WHODoRHa. World Health Organization website. http://www.who.int/rhem/prequalification/9789241599900_eng_ch1.pdf. Accessed March 24, 2020.
- Intersocietal position statement. Disinfection of ultrasound transducers used for percutaneous procedures. J Ultrasound Med 2021; 40:895–897.
- Archived – Serious Risk of Infection from Ultrasound and Medical Gels – Revision. Government of Canada website. https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2004/14289a-eng.php#. Accessed March 24, 2020.
- O'Rourke M, Levan P, Khan T. Current use of ultrasound transmission gel for transesophageal echocardiogram examinations: a survey of cardiothoracic anesthesiology fellowship directors. J Cardiothorac Vasc Anesth 2014; 28:1208–1210.
- Rutala WA, White MS, Gergen MF, Weber DJ. Bacterial contamination of keyboards: efficacy and functional impact of disinfectants. Infect Control Hosp Epidemiol 2006; 27:372–377.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020. doi: 10.105